IWILFIN and Relapse Reduction
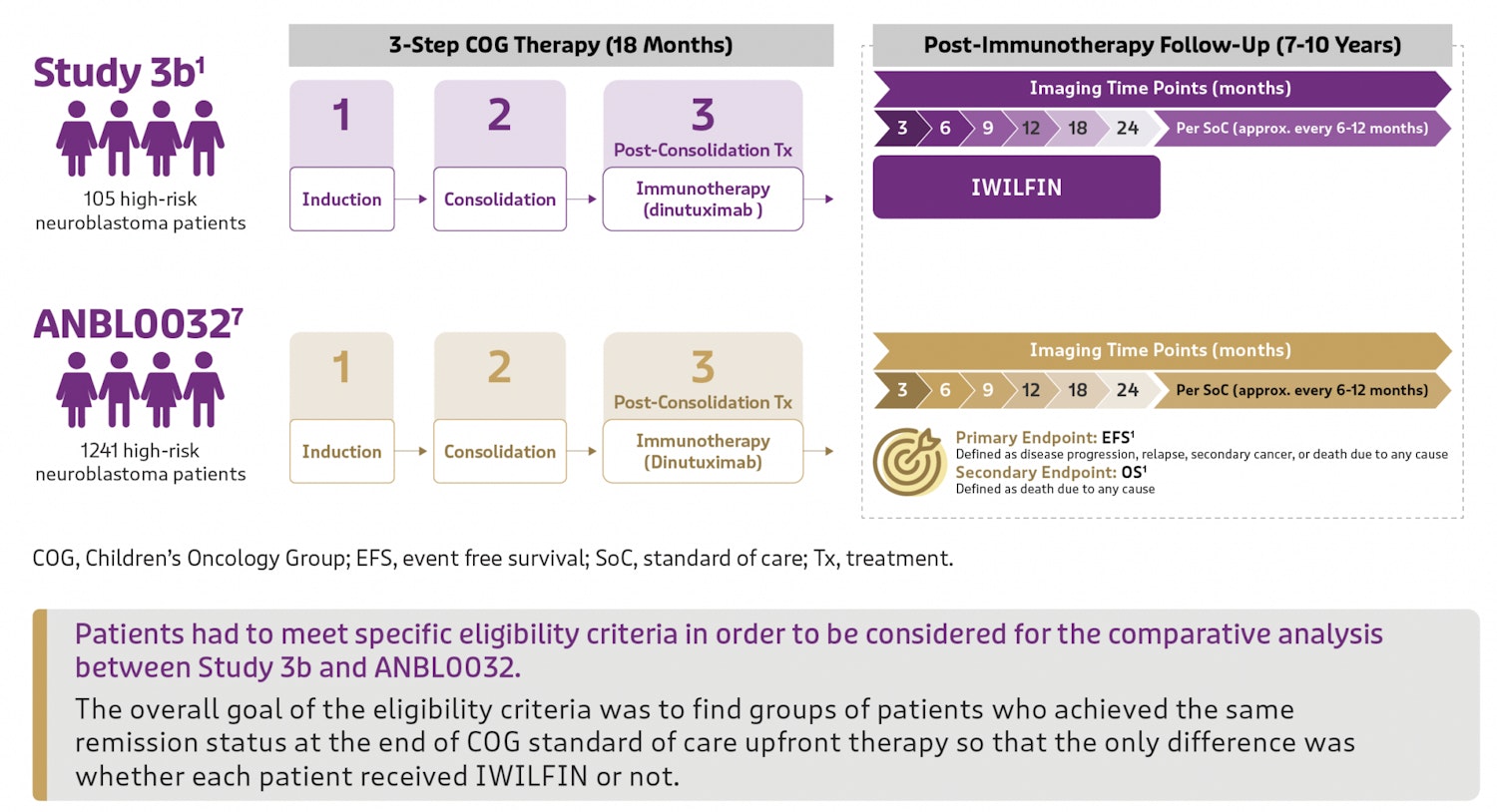
IWILFIN was assessed in Study 3b, a clinical trial examining its safety and efficacy in 105 pediatric patients with high-risk neuroblastoma. All patients received IWILFIN twice daily for up to 2 years after achieving remission from upfront therapy.
To better interpret the outcomes of Study 3b, its results were compared to an external control database of similar high-risk neuroblastoma patients from another trial—Study ANBL0032, which studied pediatric patients who also achieved remission after upfront treatment but who did not go on to receive IWILFIN.